Hematoscope Oy - The path to clinical uptake of the Hematoscope app
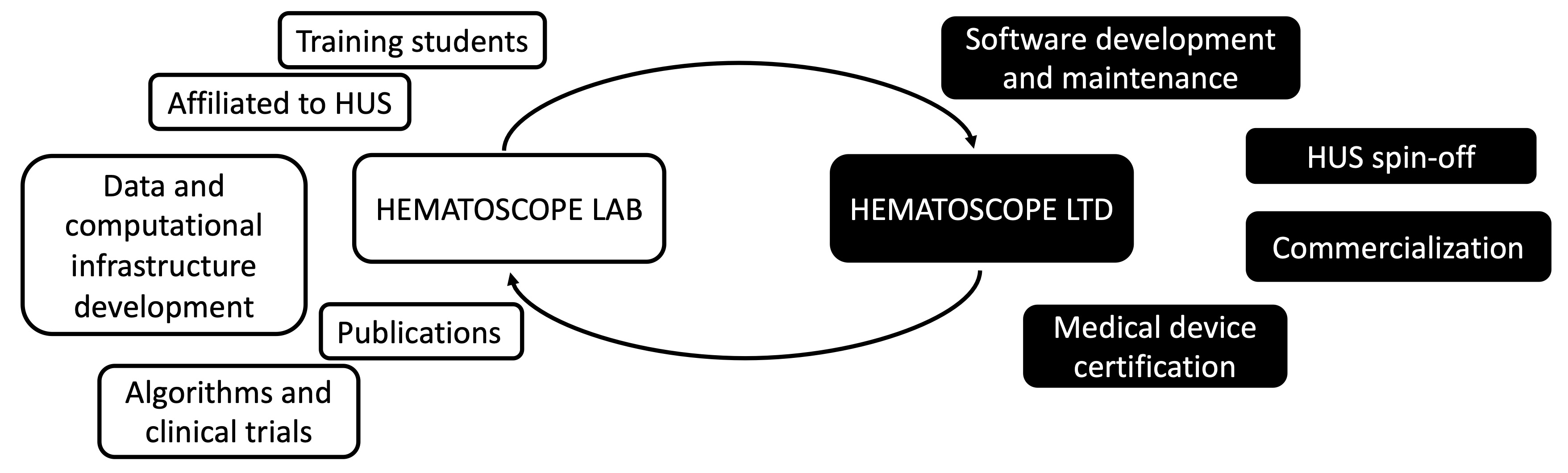
As you may be aware, our research group has been working towards making the Hematoscope app suitable for clinical use. Our focus has been on developing model infrastructures, creating algorithms, and publishing results for the scientific community. We also aim to provide a platform for students to learn. However, clinical translation requires a different set of skills, investing time and funding in robust validation, clinical quality management systems, medical device certification, and clinical practice integration. Unfortunately, academic and hospital institutes have not established infrastructures to actually translate innovations to clinical care. Therefore, these are tasks are too complex to be performed by academic teams.
To address this challenge, we have established the Hematoscope Oy, a medical software company dedicated. While these are different organizations, The Hematoscope Lab and Hematoscope Oy will have synergies - most importantly the goal to improve clinical diagnostics and care in hematology. I will be devoting my work time to heading the Hematoscope Lab. Due to my dual role, I will maintain 100% transparency in possible conflicts of interest. While we are in the early phase of the medical certification path, there will be no business activity for at least 12 months. In the long run, the Hematoscope app is committed to provide digital cytopathology diagnostic support globally, and have planned specific use cases for healthcare professionals, pharma and biotech companies, and students in laboratory/clinical medicine.
The Hematoscope Oy would also like to warmly thank the Instrumentarium Foundation for their contribution and generous funding to support our journey! Please find a complimentary interview by Kai Tarkka.
Previous post
Medical Device Regulation (MDR) Specialist
